
Fundamental Research | Effect of Sn doping concentration on the oxidation of Al-containing MAX phase
1、Research background:
With the rapid development of modern science and technology, human beings have more and more stringent requirements for material properties, and it is often difficult for a single material to meet the requirements of human use. The MAX phase has both metallic and covalent bonds, so it has the advantages of both metals and ceramics. MAX phase materials have excellent processability, good thermal and electrical conductivity, high thermal shock stability, light weight, high Young's modulus and excellent oxidation resistance. This makes the MAX phase widely used in metallurgy, aerospace, machinery and other fields.
The MAX phase is a class of layered ternary compounds composed of transition metal elements, main group elements and carbon or nitrogen. Among them, Ti3AlC2 is the lightest 312 series MAX phase found so far, and its density is 4.25 g/cm3. At the same time, Ti3AlC2 also has excellent anti-oxidation properties. However, Ti3AlC2 has a narrow phase domain in the Ti-Al-C phase diagram, and its synthetic raw material Al is easily lost by evaporation. Therefore, impurities such as TiC, Ti2AlC or TixAly intermetallic compounds will inevitably be formed during the synthesis of Ti3AlC2, thereby affecting the high temperature performance of Ti3AlC2. In order to improve the purity of Ti3AlC2, researchers tried to introduce additives such as Si, Sn and B2O3 into the Ti-Al-C system. Among them, the introduction of Sn instead of Al can effectively solve the above problems and synthesize high-purity Ti3AlC2. In addition, the incorporation of Sn can improve the flexural strength of Ti3AlC2 and reduce the synthesis temperature of Ti3AlC2. However, the oxidation resistance of Ti3AlC2 largely depends on the Al content. At the same temperature, the oxidation parabolic rate constant of 20 mol% Sn-doped Ti3AlC2 is 2~4 orders of magnitude higher than that of pure Ti3AlC2. Therefore, it is crucial to determine the appropriate Sn doping concentration range to obtain higher purity Ti3AlC2 while maintaining better oxidation resistance.
Recently, Green Metallurgy Team of University of Science and Technology Beijing used a combination of simulation and experiment to systematically study the effect of Sn doping concentration on the oxidation behavior of Ti3AlC2 from an experimental and theoretical perspective. The related conclusion "Effect of Sn doping concentration on the oxidation of Al-containing MAX phase (Ti3AlC2) combining simulation with experiment" was published in the Fundamental Research journal, which is directed and sponsored by the National Natural Science Foundation of China. According to reports, Fundamental Research is a comprehensive English academic journal that includes eight disciplinary branches. Its positioning and targets are Nature, Science, CELL, and PNAS. The research reveals the nature of the Ti3AlC2/O2 interface reaction during the oxidation process by first-principles and molecular dynamics methods. Meanwhile, according to the theoretical calculation results, when the Sn doping concentration exceeds 10 mol%, SnO2¬ in the oxidation product inhibits the formation of a protective continuous Al2O3 layer, thereby reducing the oxidation resistance of Ti3AlC2. Thermogravimetric oxidation experiments combined with the phase morphology of the materials verified the theoretical simulation results. Therefore, the oxidation mechanism of Ti3AlC2 doped with different concentrations of Sn is proposed (Fig. 1). This work provides a theoretical basis and data reference for designing MAX phase materials with high oxidation resistance.
2、Graphic guide:
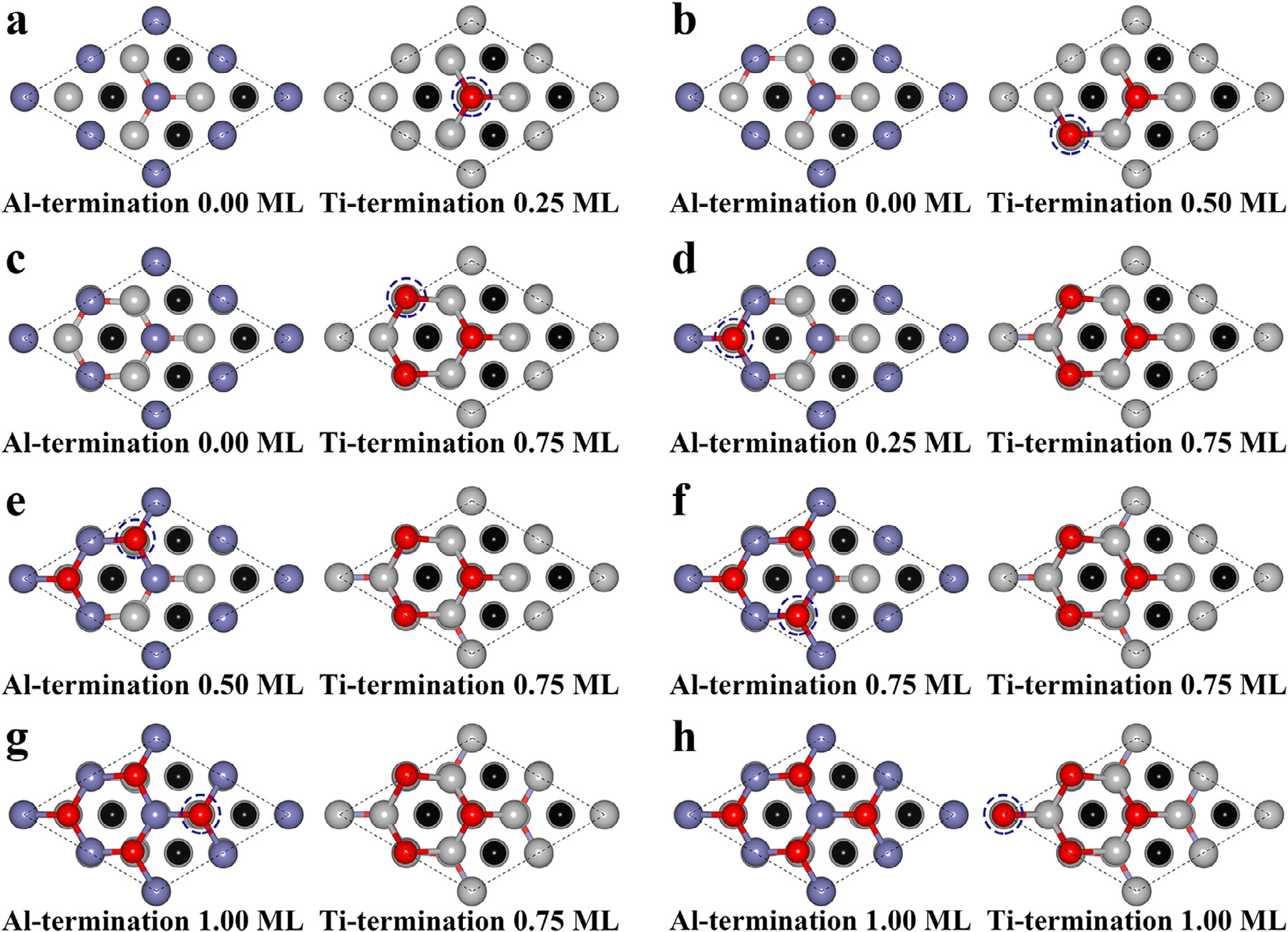
Fig1. Structures of each oxidation step of the Ti3AlC2 (100) surface.
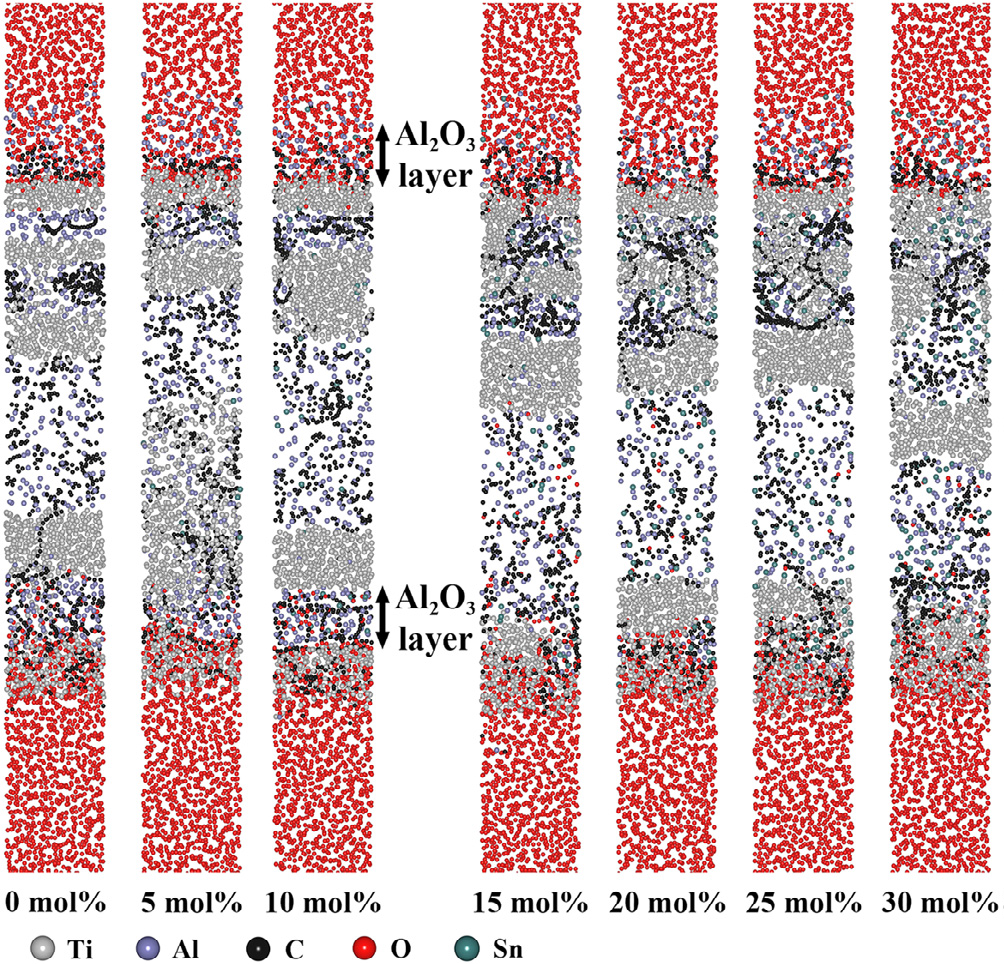
Fig. 2. Configurations of Ti3AlC2 with different Sn doping concentrations (0-30 mol% with intervals of 5 mol%) after oxidation for 50 ps at 1273 K.
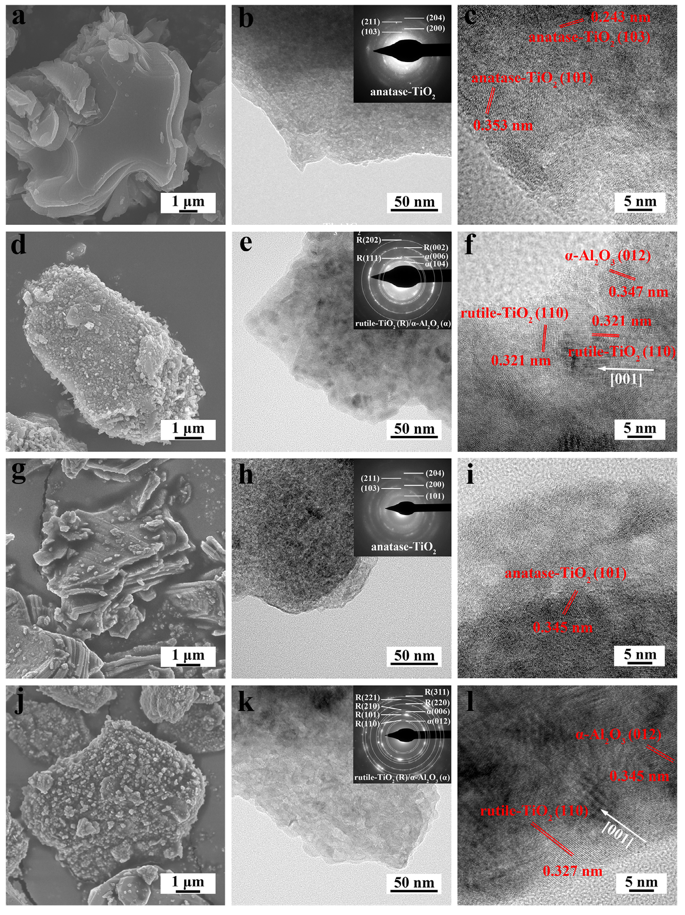
Fig. 3. SEM images, TEM images together with the corresponding SAED patterns and HRTEM images of Ti3AlC2 and Ti3Al0.8Sn0.2C2 powders oxidized at different temperatures for 10 h. (a, b, c) Ti3AlC2 powder oxidized at 500 ℃; (d, e, f) Ti3AlC2 powder oxidized at 700 ℃; (g, h, i) Ti3Al0.8Sn0.2C2 powder oxidized at 500 ℃; (j, k, l) Ti3Al0.8Sn0.2C2 powder oxidized at 700 ℃.
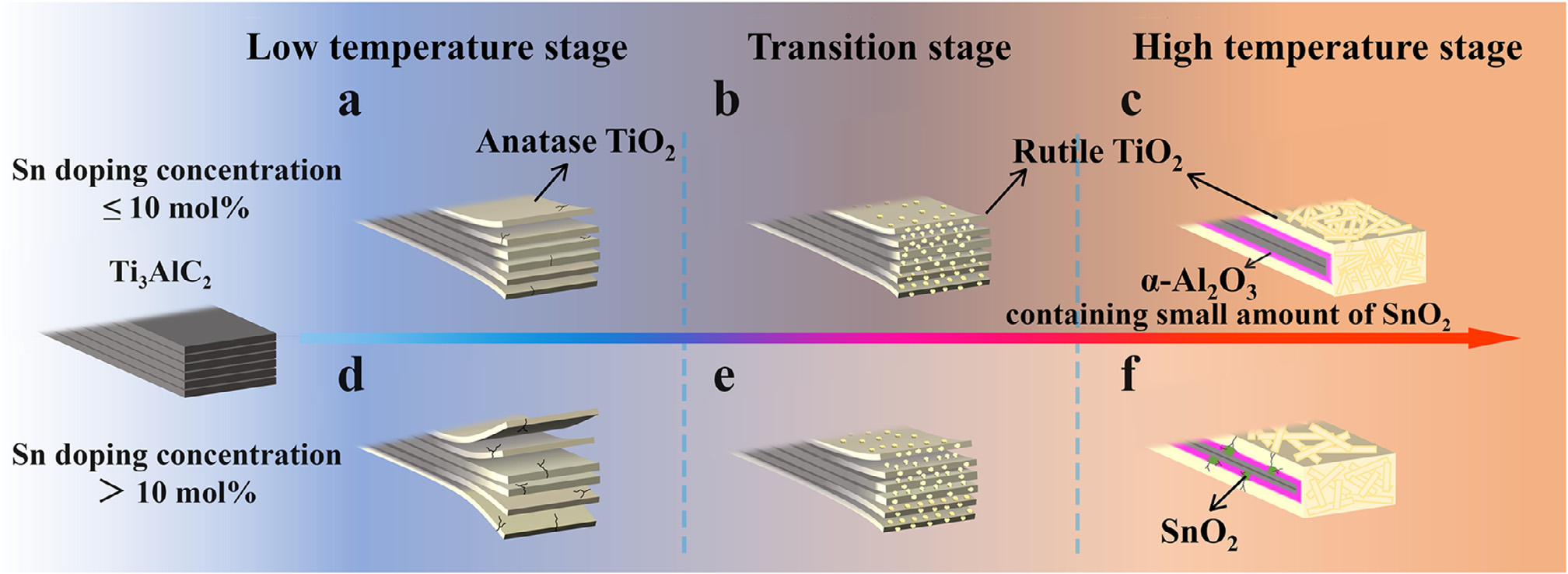
Fig. 4. Oxidation schematic diagrams of Ti 3 AlC 2 powder with different Sn doping concentrations.
Team introduction
The "Green Metallurgy" team of the University of Science and Technology Beijing Iron and Steel Generic Technology Collaborative Innovation Center is based on the theoretical basis of metallurgical physics and chemistry, breaking through the limitations of traditional metallurgy, chemical industry, energy, environment and materials, and exploring the effects of new materials in the fields of steel, energy and environment. enhancement. The main research directions of the echelon include: (1) interface reaction kinetics; (2) research on the boundary wettability of high-temperature solutions; (3) development of new high-temperature ceramic materials; (4) metallurgical process wastewater, waste liquid, flue gas and solid waste Governance and comprehensive utilization; (5) Development of new optoelectronic and power-electric functional materials and device construction, etc.; (6) Multi-structure control of functional materials based on electrochemical metallurgy. Currently, there are 3 Professors (including 1 National Distinguished Young Scholars), 1 Associate Researchers, and 1 Assistant Researcher. The team has undertaken more than 20 national, provincial and ministerial projects, published more than 200 SCI papers, applied for more than 30 patents, and won more than 10 provincial and ministerial awards.

