
N-doped carbon-coated freestanding Fe film with sea urchin-like micro/nanoporous structure for effic
N-doped carbon-coated freestanding Fe film with sea urchin-like micro/nanoporous structure for efficient oxygen evolution reaction catalyst
Xiangtao Yu*, Xiangyu Ren, Zhangfu Yuan, Mingyong Wang, N-doped carbon-coated freestanding Fe film with sea urchin-like micro/nanoporous structure for efficient oxygen evolution reaction catalyst, Functional Materials Letters, (2021), 2150037, DOI: 10.1142/S1793604721500375
With the increasing depletion of fossil energy and stricter environmental protection measures, clean energy represented by hydrogen energy has become increasingly popular. Hydrogen production by water electrolysis driven by solar and wind energy is considered to be a promising technology. Due to the slow kinetics of the four-electron transfer process, the oxygen evolution reaction (OER) is the bottleneck of water electrolysis for hydrogen production. It is necessary to prepare an efficient, inexpensive and stable OER electrocatalyst. The earth's abundant transition metal Fe has been widely studied as a potential noble metal OER electrocatalyst substitute. It is believed that electronic interaction between the N-doped carbon coating and Fe will cause the binding energy species of *OOH and *OH adsorbed on the surface to be closer to the optimal value of 0. In addition, N-doped carbon coating can improve conductivity. Therefore, the N-doped carbon-coated Fe film has high intrinsic OER catalytic activity.
Recently, the “green metallurgy” research team led by Professor Hou Xinmei of University of Science and Technology Beijing published a research paper titled “N-doped carbon-coated freestanding Fe film with sea urchin-like micro/nanoporous structure for efficient oxygen evolution reaction catalyst” and proposed The sea urchin-like structure is directly constructed on the surface of the 3D porous metal film through the MOFs reaction, and the N-doped carbon-coated micro-nano urchin-like porous electrode is constructed by anaerobic pyrolysis and used as the oxygen evolution electrode for hydrogen production by water electrolysis. The electrode exhibits good hydrogen evolution activity and stability. The results were published in the international journal "Functional Materials Letters".
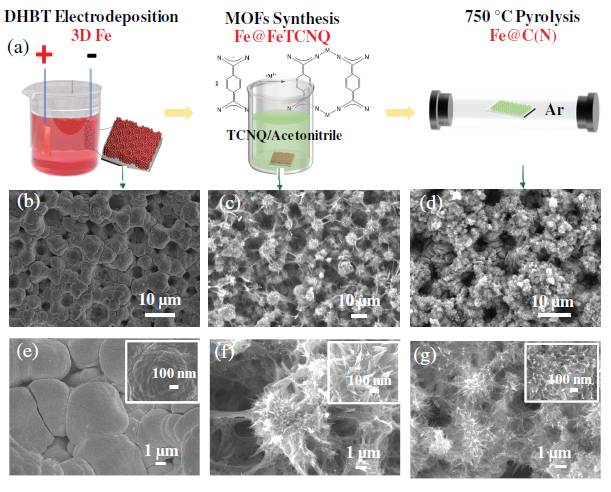
Figure 1 Schematic diagram of preparation process and evolution of morphology and structure of N-doped carbon-coated Fe electrode (Fe@C(N)).
This article first prepared a 3D porous Fe self-supporting film by hydrogen bubble template electrodeposition; then the film was immersed in TCNQ/acetonitrile solution for reaction, and the reaction was controlled by controlling the concentration of organic matter, reaction temperature and reaction kinetic conditions (stirring), etc. The growth of the product finally obtains a sea urchin-like MOFs structure; finally, the reaction product is subjected to anaerobic pyrolysis to obtain an N-doped C-coated self-supporting porous membrane with a micro-nano porous structure. Through the characterization and analysis of X-ray diffraction and transmission electron microscopy, the product was determined to be Fe@C(N). The self-supporting porous membrane of sea urchin-like Fe@C(N) micro-nano porous structure coated with self-supporting N-doped carbon as an oxygen evolution catalytic electrode exhibits good OER catalytic activity and stability. Fe@C is measured at room temperature (N) The Tafel slope of the film in 1 M NaOH and the OER overpotential at 10 and 200 mA cm−2 are 75 mV dec−1, 77 and 453 mV, respectively, which are much lower than the 3D porous Fe film (129 mV dec− 1, 108 and 595 mV).
Finally, this article discusses the reasons for the high oxygen evolution activity and stability of the N-doped carbon-coated sea urchin-like Fe@C(N) self-supporting micro-nano porous membrane. First of all, the sea urchin-like micro-nano porous membrane has good wettability, which can promote the generation of oxygen bubbles from the electrode surface in time, and the structure has a large active specific surface area that is conducive to ion transmission. Secondly, the nitrogen-doped carbon coating structure can prevent oxidation of the anode and enhance conductivity. Third, electronic interactions between the N-doped carbon coating and Fe will cause the binding energy species of *OOH and *OH adsorbed on the surface to be closer to the optimal value. Finally, the multi-structure synergistic effect enhances the oxygen evolution activity and stability of the electrode.
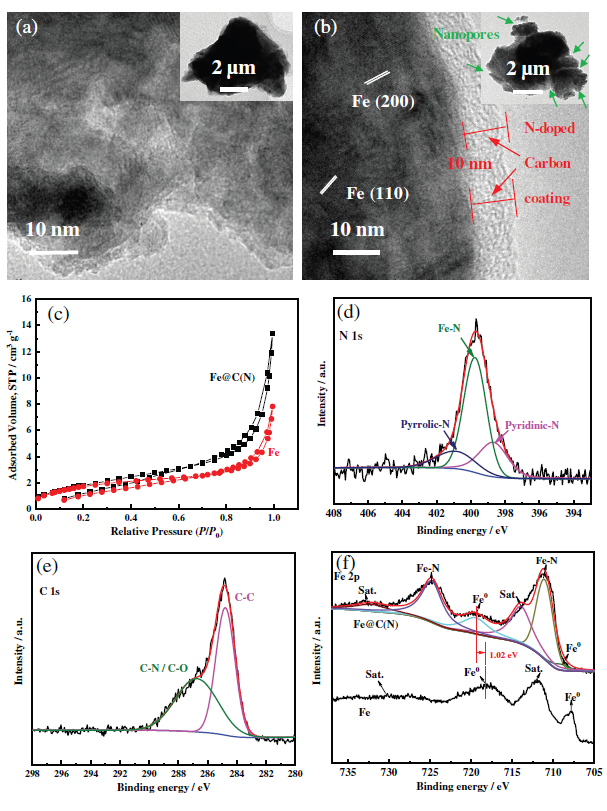
Figure 2 Characterization of structure and composition of N-doped carbon-coated Fe@C(N) electrode.

Figure 3 Characterization of oxygen evolution performance of N-doped carbon-coated Fe@C(N) electrode.
The "Green Metallurgy" team of the University of Science and Technology Beijing Iron and Steel Generic Technology Collaborative Innovation Center is based on the theoretical basis of metallurgical physics and chemistry, breaking through the limitations of traditional metallurgy, chemical industry, energy, environment and materials, and exploring the effects of new materials in the fields of steel, energy and environment. enhancement. The main research directions of the echelon include: (1) interface reaction kinetics; (2) research on the boundary wettability of high-temperature solutions; (3) development of new high-temperature ceramic materials; (4) metallurgical process wastewater, waste liquid, flue gas and solid waste Governance and comprehensive utilization; (5) Development of new optoelectronic and power-electric functional materials and device construction, etc.; (6) Multi-structure control of functional materials based on electrochemical metallurgy. Currently, there are 3 Professors (including 1 National Distinguished Young Scholars), 1 Associate Researchers, and 1 Assistant Researcher. The team has undertaken more than 20 national, provincial and ministerial projects, published more than 200 SCI papers, applied for more than 30 patents, and won more than 10 provincial and ministerial awards.

The research group of professor Xinmei Hou

The research group of professor Zhangfu Yuan

